- Get link
- X
- Other Apps
1 ZEPOSIA an oral medication taken once daily. Received its first approval on 25 March 2020 in the USA.
 Ozanimod Gets Priority Review For Ulcerative Colitis Mpr
Ozanimod Gets Priority Review For Ulcerative Colitis Mpr
BMY today announced that the US.

Ozanimod ulcerative colitis approval. If approved Zeposia would be the first oral sphingosine-1-phosphate S1P receptor modulator for the treatment of ulcerative colitis. Patients could not have known strictures or stenosis leading to symptoms of obstruction current stoma or need for ileostomy or colostomy extensive. Supplemental New Drug Application is supported by positive results from the pivotal Phase 3 True North study evaluating oral Zeposia ozanimod in adults with moderately to severely active ulcerative colitis.
Bristol-Myers Squibb Company NYSE. Small ulcers can develop on the colons lining which can. Food and Drug Administration has approved ozanimod an immune-modulating therapy invented at Scripps Research for the treatment of adults with relapsing forms of multiple sclerosis.
The drug is also in advanced clinical development for. FDA-APPROVED INDICATION FOR ZEPOSIA ZEPOSIA is indicated for the treatment of. Ozanimod for treating moderately to severely active ulcerative colitis ID3841 Proposed GID-TA10732 Expected publication date.
Orally-active S1P agonist Zeposia ozanimod has been submitted to the FDA as a treatment for adults with moderately to severely active ulcerative colitis UC with a. The trial was not large enough or of sufficiently long duration to establish clinical efficacy or assess safety. Patients with ulcerative colitis indeterminate colitis or Crohns disease isolated to the stomach duodenum jejunum or peri-anal region without colonic or ileal involvement were excluded.
Ozanimod is currently in clinical development for adult patients with moderate to severe ulcerative colitis UC. Ozanimod Treatment for Ulcerative Colitis U lcerative colitis is a chronic immune-mediated disease of the colon that is currently treated with. Ozanimod ZEPOSIA.
In a placebo-controlled phase 2 trial involving patients with ulcerative colitis ozanimod an oral agonist of sphingosine-1-phosphate receptor subtypes 1 and 5 resulted in a slightly higher rate. Ozanimod is also being studied as a treatment for forms of inflammatory bowel disease with late-stage clinical trials underway for. Zeposia is not approved for the treatment of ulcerative colitis in any country.
Late-stage ozanimod trials are ongoing in ulcerative colitis and Crohns disease. Celgene Corporation is a novel orally administered sphingosine 1-phosphate S1P receptor modulator. Bristol Myers Squibb this week announced the positive results from its pivotal clinical trial for ulcerative colitis saying ozanimod demonstrated a consistent clinical benefit among patients who took the once-daily oral therapy for.
Zeposia has been approved by the FDA on the strength of the phase 3. Food and Drug Administration FDA approved ZEPOSIA ozanimod 092 mg for the treatment of adults with relapsing forms of multiple sclerosis RMS including clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease. Ozanimod binds to and internalizes the S1P subtype 1 receptor preventing certain proinflammatory lymphocytes from exiting the lymph nodes and circulating to the intestinal tissue.
An S1P receptor modulator is being developed by Celgene a Bristol-Myers Squibb Company for the treatment of multiple sclerosis ulcerative colitis and Crohns disease. LA JOLLA CA The US. Celgene was acquired by Bristol Myers Squibb in 2019.
Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis In this preliminary trial ozanimod at a daily dose of 1 mg resulted in a slightly higher rate of clinical remission of ulcerative colitis than placebo. Ulcerative colitis is a long-term condition where the colon and rectum parts of the bowel become inflamed. The drug ozanimod which is being developed by Bristol Myers Squibb under the name Zeposia gained FDA approval earlier this year for multiple sclerosis.
Ozanimod is the latest in a series of novel medicines to originate from the laboratories of Scripps Research. In March 2020 the US FDA approved ozanimod capsules for use in the treatment of relapsing forms of multiple sclerosis to include clinically isolated syndrome relap.
 Ozanimod Accepted For Priority Review By Fda For The Treatment Of Ulcerative Colitis Scripps Research
Ozanimod Accepted For Priority Review By Fda For The Treatment Of Ulcerative Colitis Scripps Research
 An Overview Of Novel And Emerging Therapies For Inflammatory Bowel Disease European Medical Journal
An Overview Of Novel And Emerging Therapies For Inflammatory Bowel Disease European Medical Journal
 Ozanimod Induction Therapy For Patients With Moderate To Severe Crohn S Disease A Single Arm Phase 2 Prospective Observer Blinded Endpoint Study The Lancet Gastroenterology Hepatology
Ozanimod Induction Therapy For Patients With Moderate To Severe Crohn S Disease A Single Arm Phase 2 Prospective Observer Blinded Endpoint Study The Lancet Gastroenterology Hepatology
 Ulcerative Colitis Today Tomorrow And The Future European Medical Journal
Ulcerative Colitis Today Tomorrow And The Future European Medical Journal
 Ozanimod Induction And Maintenance Treatment For Ulcerative Colitis Nejm
Ozanimod Induction And Maintenance Treatment For Ulcerative Colitis Nejm
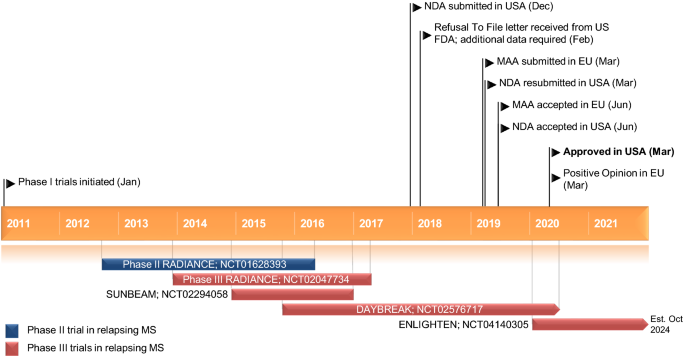 Ozanimod First Approval Springerlink
Ozanimod First Approval Springerlink
Bolder Science Ozanimod S1p1 And S1p5 Modulator
 True North Ozanimod Phase Iii In Ulcerative Colitis Uc Completed Enrollment Celg Message Board Posts
True North Ozanimod Phase Iii In Ulcerative Colitis Uc Completed Enrollment Celg Message Board Posts
 True North Ozanimod Phase Iii In Ulcerative Colitis Uc Completed Enrollment Celg Message Board Posts
True North Ozanimod Phase Iii In Ulcerative Colitis Uc Completed Enrollment Celg Message Board Posts
 Ulcerative Colitis Today Tomorrow And The Future European Medical Journal
Ulcerative Colitis Today Tomorrow And The Future European Medical Journal
 Ozanimod Induction And Maintenance Treatment For Ulcerative Colitis Nejm
Ozanimod Induction And Maintenance Treatment For Ulcerative Colitis Nejm
 Small Molecule Oral Targeted Therapies In Ulcerative Colitis The Lancet Gastroenterology Hepatology
Small Molecule Oral Targeted Therapies In Ulcerative Colitis The Lancet Gastroenterology Hepatology
 In Pivotal Phase 3 Trial Ozanimod Demonstrates A Clear Benefit Among Patients With Moderate To Severe Ulcerative Colitis Scripps Research
In Pivotal Phase 3 Trial Ozanimod Demonstrates A Clear Benefit Among Patients With Moderate To Severe Ulcerative Colitis Scripps Research

Comments
Post a Comment